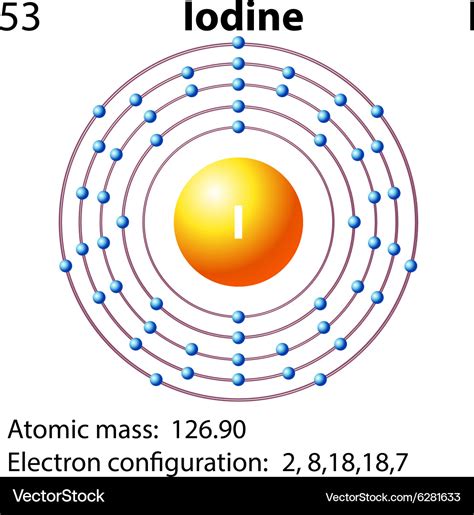
iodine electron arrangement|Iodine Electron Configuration (I) with Orbital Diagram : Tagatay The total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. . Tingnan ang higit pa Travel like a local wherever you're going! Connect with Local Experts based in your destination for insider tips, hidden gems, local recommendations, and expert itinerary advice. Plan your trip with a local, in-destination travel agent and discover unique and authentic experiences.
PH0 · WebElements Periodic Table » Iodine » properties of free atoms
PH1 · WebElements Periodic Table » Iodine » properties of
PH2 · Iodine Electronic Configuration and Distribution in Shells
PH3 · Iodine Electron Configuration: Everything You Need to Know
PH4 · Iodine Electron Configuration (I) with Orbital Diagram
PH5 · Iodine (I)
PH6 · Iodine
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · Complete Electron Configuration for Iodine (I, I– ion)
PH9 · 2.4 Electron Configurations
PAGCOR oversees the Philippine gaming industry through strict regulation of all persons, locations, practices and related activities. Other than land-based casinos, there are licensed gaming sites around the country offering Poker Games. . APPLICATION. Application for a gaming license to operate a Poker club follows a structured two (2)-part .
iodine electron arrangement*******Atoms can jump from one orbital to another orbital in an excited state. This is called quantum jump. The ground state electron configuration of iodine is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p5. In the iodine ground-state electron configuration, the last electrons of the 5p orbital are located in the . Tingnan ang higit pa
The total number of electrons in iodine is fifty-three. These electrons are arranged according to specific rules in different orbitals. . Tingnan ang higit paScientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the . Tingnan ang higit paAfter arranging the electrons, it is seen that the last shell of the iodine atom has seven electrons. Therefore, the valence electronsof iodine are seven. The elements . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa
Mar 23, 2023 Electron configuration: The arrangement of electrons in an atom or molecule. Melting point: The temperature at which a solid substance turns into a liquid. .
Iodine Number of Valence Electrons. Iodine has seven Valence Electrons. What is The Electron Configuration of Iodine. There are 53 electrons in iodine that occupy the respective orbitals as given . This electron arrangement and electron configuration indicates that the outermost orbit (i.e orbit number 5) of an iodine atom has 7 electrons. Hence, it lies in .iodine electron arrangement Iodine Electron Configuration (I) with Orbital DiagramElectron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. .a) Find the electron configuration of iodine [Kr] 5s 2 4d 10 5p 5. b) How many unpaired electrons does iodine have? To find the answer we refer to part a) and look at the valence electrons. We see that iodine has 5 . In this article, we will study how electrons are arranged in different shells and subshells in the Iodine atom. Table of Contents. 1. Iodine. 2. Electronic Configuration of . Iodine is a 5-period element with a total of 53 electrons, filled in the ascending series of energy levels 1s, 2s, 2p, and so on. The s, p, d and f orbitals can hold a .
Iodine atoms have 53 electrons and the shell structure is 2.8.18.18.7. The ground state electron configuration of ground state gaseous neutral iodine is [ Kr ]. 4d10. 5s2. 5p5 and the term symbol is .
Iodine is the only solid halogen at room temperature. It forms a purple gas when heated, and bears the Greek name iodine (violet). . The electron configuration of an element describes the arrangement of electrons in the atoms of that element, and be used to predict its chemical properties and reactivity. In the electron configuration notation .
Iodine atoms have 53 electrons and the shell structure is 2.8.18.18.7. The ground state electron configuration of ground state gaseous neutral iodine is [Kr].4d 10.5s 2.5p 5 and the term symbol is 2 . The electronic configuration of an element is used to describe the arrangement of its electrons in its atomic orbitals. Let us discuss the energy levels that. . Ground state Iodine electron configuration. The ground state electrical configuration is I is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 5.This means the first shell (1s) has 2 electrons. The second shell (2s and 2p) has a total of 8 electrons. And, the third (last) shell has 1 electron. The first and second shells comprise the core (inner) electrons = 2 + 8 = 10 electrons. The outermost (valence) has 1 electron. The shell diagram of the Na atom is shown here.
In the iodine orbital diagram, the 1s subshell accommodates two electrons, the 2s subshell carries another pair, the 2p subshell encompasses six electrons, the 3s subshell contains two electrons, the 3p subshell carries six electrons, the 4s subshell holds two electrons, the 3d subshell carries ten electrons, the 4p subshell .Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2 p 5; Electrons per Energy Level: 2,8,18,18,7 Shell Model; . Iodine - I (EnvironmentalChemistry.com)- Comprehensive information for the element Iodine - I is provided by this page including scores of properties, element names in many languages, most known nuclides and .Iodine (I) element properties, information, facts, uses and Periodic Table trends. Complete information about the Iodine element - Atomic Number 53, atomic mass [126.90447], melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron .The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Iodine is 53. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.Iodine has 53 electrons and a configuration of 2, 8, 18, 18, 7. Why does the fourth shell not reach maximum and therefore it picks up 7 electrons and begins a new shell? Electronic configuration. Filling of electrons in atomic orbitals takes place according to Aufbau principle, Pauli's exclusion principle and Hund's rule of maximum multiplicity.
Valence electrons are the outermost electrons of an atom, and they play a crucial role in chemical bonding. The electron dot diagram of iodine shows the arrangement of its valence electrons. In the case of iodine, the electron dot diagram would depict the symbol “I” surrounded by dots that represent its seven valence electrons.
Iodine is the fourth halogen, being a member of group 17 in the periodic table, below fluorine, chlorine, and bromine; since astatine and tennessine are radioactive, iodine is the heaviest stable halogen. Iodine has an .
Iodine, complete electron configuration. © 2009-2016 | www.prvky.com | kontaktkontakt
Electron shells have one or more electron subshells, or sublevels. The name for electron shells comes from the Bohr model, in which groups of electrons were believed to go around the nucleus at certain distances, so that their orbits formed "shells". An electron shell may be thought of as an orbit followed by electrons around an atom . Iodine i (element 53) of periodic tableIodine electron configuration atom The top shows the arrangement of iodine atoms at the surface of a layerIodine electron. Iodine electron configuration (i) with orbital diagramFacts about iodine Configuration electron iodine diagram orbital periodictable valence electrons kryptonLewis if3 .

The first step is to count all the valence electrons of each molecule. In the case of IF5, The Iodine atom has 7 valence electrons. F also has 7 valence electrons. But since there are 5 atoms of F, we multiply 7×5= 35 valence electrons. Adding both we get 35+7= 42. Hence, a total number of valence electrons of IF5= 42. 2. Determining the .
Because electrons repel each other electrostatically, the most stable arrangement of electron groups (i.e., the one with the lowest energy) is the one that minimizes repulsions. . The central atom, iodine, contributes seven electrons. Each chlorine contributes seven, and there is a single negative charge. The Lewis electron .Iodine Electron Configuration (I) with Orbital Diagram An explanation of the molecular geometry for the IF3 (Iodine trifluoride) including a description of the IF3 bond angles.The ideal bond angle for the Iodine .iodine electron arrangementElement Iodine (I), Group 17, Atomic Number 53, p-block, Mass 126.904. . Members of a group typically have similar properties and electron configurations in their outer shell. . images may not be posted on any website, shared in any disc library, image storage mechanism, network system or similar arrangement. Pornographic, defamatory .
Table games dealers operate casino gaming tables at licensed casinos and gaming halls. Table games dealers receive extensive in-house training and are required to shuffle and deal cards, hand out .
iodine electron arrangement|Iodine Electron Configuration (I) with Orbital Diagram